A Nebraska Medicine patient becomes first in the world to receive new MS treatment
A Nebraska Medicine patient becomes first in the world to receive new MS treatment
By Kassidy Arena , Senior Reporter Nebraska Public Media News
Aug. 5, 2025, 1:54 p.m. · 3 min read
Nebraska Medicine is offering a first-of-its-kind clinical trial for a new multiple sclerosis (MS) treatment. MS affects a person’s central nervous system.
The new treatment, which has been used for blood cancers, would genetically modify healthy T-cells to attack the cells believed to cause the autoimmune attacks of MS.
“What's exciting about this treatment is that potentially, CAR T therapy might turn [out] to be the treatment that can address an important treatment gap in multiple sclerosis, which is progressive MS,” said Dr. Rana Zabad, who runs the Nebraska Medicine multiple sclerosis program.
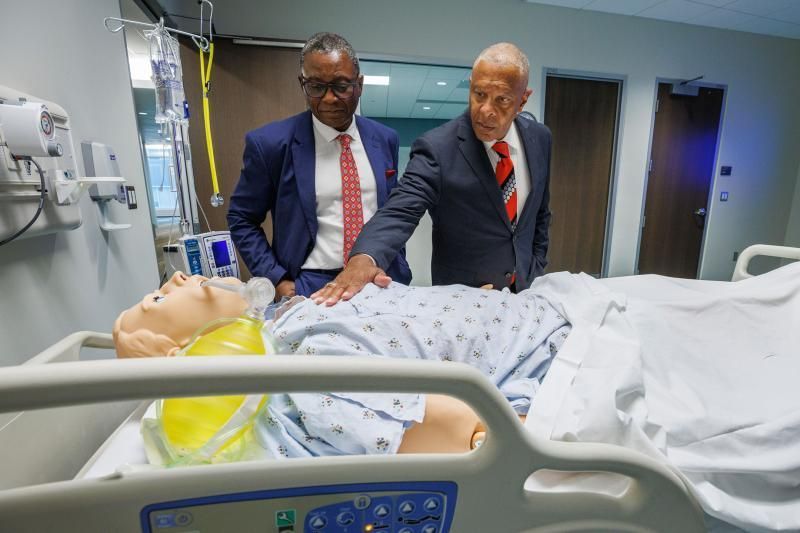
Jan Janisch-Hanzlik is the first person in the world to receive this new treatment called allogeneic CAR T-Cell therapy. The treatment utilizes a healthy person’s donated T-cells, which are then genetically modified to take out the cells believed to cause the autoimmune attacks associated with MS. It’s been used before to treat blood cancers, but this is a first-of-its-kind trial for MS treatment.
Janisch-Hanzlik said she is feeling great after the therapy, which followed a round of chemotherapy.
“I can run faster and jump higher, but I couldn't run before, and probably still can't run now, but I definitely can jump,” she said with a laugh. “I never ever thought that my journey with MS would embark on something so groundbreaking.”
According to the National Multiple Sclerosis Society, MS affects almost three million people worldwide.
“I wanted to do it, not just for me, but really, for the whole MS population,” Janisch-Hanzlik added.
Nebraska Medicine doctors said they are cautiously optimistic this new treatment could potentially bring down that number, but they are only in phase one of the treatment.
Dr. Matthew Lunning, the medical director of gene and cellular therapy, said the technology used in this therapy isn’t new, but the application of it for MS treatment is. The allogeneic, meaning donated, CAR T-cells are genetically enhanced and manipulated to go after a B cell called CD 19.
“The hope is that, over time, those B cells will reset,” he said. “Kind of like restarting a computer: control, alt, delete, that allows those B cells to come back, but come back as better B cells, and not the B cells that were doing the harm.”
Zabad clarified this is not a cure, because there are several more stages of the treatment in order to medically determine efficacy.
Nebraska Medicine does have a line of patients waiting to receive this treatment, but there are a number of FDA safeguards to work through first.
As of now, Janisch-Hanzlik said she is feeling so much better that she was able to do a little gardening on her own, much to the chagrin of Zabad, who would have preferred she stay away from Nebraska’s intense summer heat.